

Normal Hours of Operation Mon-Fri: 8am-4.00pm
Information About Plainsman Products Services & Information ClaysLow Temperature ClaysMedium Temperature Clays High Temperature Clays Porcelains Other Clays Native Clays Casting Slips MaterialsDry MaterialsStains Encapsulated Stains Liquids GlazesSpectrum Opaque Gloss Low Fire GlazesSpectrum Metallic Glazes Medium Fire Glazes Plainsman Dry Glazes Potter's Choice Cone 5/6 Glazes Celadon Cone 5/6 Glazes Amaco Satin Matte Glazes Liquid Brights UnderglazesAmaco Velvet UnderglazesEnamellingEquipmentKilnsElectric Pottery KilnsElectric Glass Kilns Kiln Furniture Cones Elements Kiln Parts, Accessories Exhaust Systems Refractories Potter's Wheels Slab Rollers Hand Extruders Pugmills Scales Banding Wheels Air Brushes ToolsBrushesThrowing Tools Trimming, Turning, Cutting Tools Wood/Bamboo Tools Rollers/Stamps Decorating Tools Glazing Tools Ribs & Scrapers Ribbon/Wire Tools Rasps Knives, Needle Tools, Cutters Tool Kits Cleanup, Incising, Finishing Tools Unclassified AccessoriesMiscellaneous AccesoriesCorks/Stoppers Cork Pads Oil Lamp Accessories Dispenser Pumps Teapot Handles Bisque Tiles | Visit Our Full Catalog & Store We are one of about 90 ceramic suppliers in North America. But unlike almost all of them, we make most of our products from clays we mine and process ourselves. We intend to leverage this advantage to achieve the unmatched quality, assurance of supply and low prices this enables. Technical Tips BlogDIY the commercial glaze on mug #1:You must consider five factors to make it work
The mug on the left, #1, is a commercial brushing glaze. It is opaque enough to cover this red-burning clay body. It shows the desired effect. That depends on the fact that opaque glazes stretch thinner on the sharp edges of incised designs. If they have enough melt mobility and are applied right, the effect is amplified. This potter is attempting to mix her own DIY equivalent as a dipping glaze, adding 4% tin oxide to a transparent base glaze in #2 and zircon (a higher percentage) in #3. As you can see, the effect is not working as well, and there are several reasons: Thursday 26th February 2026 Multilayer crawling
Oil-spot effects depend on being able to layer glazes. Normally, a black underneath and matte white over top. Using dipping glazes is obviously advantageous for this type of piece; the second dip covers the other half and creates the double layer needed. But dipping glazes contain clay in the recipe (almost always kaolin or ball clay), it satisfies multiple needs: It suspends the slurry, hardens the drying glaze, and supplies critical Al2O3 to the chemistry. The upper glaze here is a matte; so it needs extra Al2O3, which means it likely has extra clay. 20% kaolin (non-plastic like EPK, Grolleg, NZ) is about the maximum or the glaze will shrink too much when drying. If extra Gerstley Borate is added, then it will be worse. Drying cracks are the result when the fragile body-bond of the lower one fails to withstand the stresses of being rewetted and tugged upon by the upper layer being applied and drying. When the cracked double-layer begins to melt, it pulls itself into islands, leaving bare body between. That is called "crawling". Context: Glaze Layering, Crawling Tuesday 17th February 2026 Tile stacking in an electric kiln - Fingers crossed!
Small-scale operations everywhere are making tile like this. Most use plastic clay intended for pottery, which introduces more drying shrinkage, complicating drying them flat. Stacking them in the kiln can be a game of chance. Stacked too tightly and they crack (mostly because of quartz inversion). Stacked to loosely and most of the energy goes into heating the shelves and stackers. Using a clay with minimal large quartz particles is the best way to avoid dunting, however that is also a balance since such clays are more difficult to fit glazes to (without crazing). Context: Tile having angular shape.., An unevenly cooled tile.., It possible to make.., A plastic pottery clay.. Tuesday 17th February 2026 Stains are better in black DIY glazesUse 5% stain instead of 15% metal oxides
Consider the hazards and hassles before choosing a black matte or gloss recipe that has high individual or combined percentages of manganese dioxide, cobalt or nickel. Context: Ceramic Stain Toxicity Label.., Two cone 6 black.., Heres evidence that using.., Ceramic Stain, Toxicity Monday 16th February 2026 A plastic pottery clay for rolling ceramic tile:Not a crazy idea when it can do what this can!
This is the dolomite body recipe L4410P (a development version of Plainsman Snow). It is monoporosa tile on steroids; this body has zero percent firing shrinkage at cone 04! Predicting the final size and keeping that size consistent is much easier with such bodies. I have measured its drying shrinkage as 6% (doing our standard SHAB test). The final size needed is 20.5 cm square. Thus, I calculate the cut size to be 20.5 / (100 - 0.06) = 21.8 cm (or 20.5 / 0.94 = 21.8 cm). To keep these flat, we put them between sheets of drywall; the process takes 2-3 days. Since no change in size occurs during firing, this body has another big advantage: Tiles stay flatter during firing (a major problem with tile production). While making wall tile using a plastic pottery body is not something for industry (especially because of the space requirements for drying), for artisans working on a small scale, a body made by mixing super plastic ball clay with dolomite produces amazing working and tactile properties. The bonus is that they work so well at low temperatures, where there are so many glazing options. Context: Monoporosa or Single Fired.., QRCode mounted on Plainsman.., Tile stacking in an.., Ceramic Tile Sunday 15th February 2026 This boron blue effect depends on three things:A dark body, variations in thickness, the right chemistry
This is G2826A3, a transparent amber glaze at cone 6 on white (Plainsman M370), black (Plainsman 3B + 6% Mason 6666 black stain) and red (Plainsman M390) stoneware bodies. When the glaze is thinly applied, it is transparent. But at a tipping-point-thickness, it generates boron-blue that transforms it into a milky white. Glazes that are very glassy but on the edge of structural instability do this. So they are not good for functional ware. Context: A pottery glaze that.., Boron Blue, Glaze thickness Saturday 14th February 2026 I Tested a Found-Clay:Was it suitable for pottery?
Would you like to be able to use your own found-clays, ones native to your area or even your property, in your production? Follow me as we evaluate a mystery clay sample provided by a potter who wants to do exactly this. I will use ordinary tools that any potter either already has or can buy at low cost. We will describe this clay in terms of plastic clay bodies and common ceramic materials that most potters already use. The potter who submitted it has worked enough with the material to suspect it has potential and he wants to know how to best utilize it (e.g. at what temperature, with what glazes, mixed with what, processed in what way). In technical terms what we are doing is called "characterization". Context: Evaluating a clay's suitability.. Thursday 12th February 2026 Tile that is "actually HANDMADE"
This artisan, Dennis Cuku, is the king of DIY tile, making "actually HANDMADE" product using a red-burding terra-cotta-like middle temperature clay body. He also makes glazes in-house and fires using 36 shapes. He mixes 129 glazes and produces about 50,000 ft.² of tile per year. Tile making presents many unique challenges, not the least of which is the need for consistency and predictability of surface character and color. This endeavour is made possible with data, a lot of it. Not just glaze recipes, but many forming, glazing and firing procedures and techniques that must be documented. Context: Potters can learn from.., An example of how.. Saturday 7th February 2026 Fine-tuning the thixotropy of a glaze or engobeFor dipping, this is so much better!
Watch this 30-second video to see. Gelled (thixotropic) slurries for dipping are so much better to work with; you'll never go back once you have mastered this DIY technique. While some glazes and engobes gel naturally, especially those with high clay content, these almost always work best when the water content is within a certain range, so fine-tuning like this is still needed. Although not shown here, if over-gelling happens, a drip or two of deflocculant (e.g. Darvan) brings back the fluidity, this is more likely to happen with engobes since they need more gel (for dipping and even more for painting). A side benefit of this: No settling in the bucket. Context: Fine tune the thixotropy.. Wednesday 4th February 2026 Phase separation close-upThe power of modern phone cameras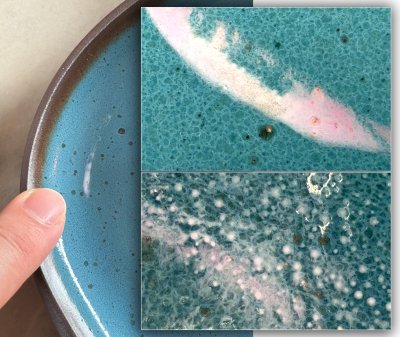
This reduction stoneware glaze is producing white streaks on some pieces (left center). The body is a coarse iron stoneware. A magnification is needed to better explain this. Context: New macro-capable cameras on.. Friday 30th January 2026 Cone 6 iron red with a catcher glaze
This is G3948A (similar to the popular Amaco Ancient Copper product). To get this stunning result, it needs to be applied thickly. Therefore, it runs a lot. But the catcher glaze around the bottom of these mugs has stopped the flow. The catcher is a glossy black, G3914A (but Amaco Obsidian would also likely work). I have learned to put it on with the right height (about 2cm) and right thickness, and then apply wax emulsion to prevent the iron red glaze from sticking during dipping. The inside glaze, G2926B, is one I have tested and developed to fit Plainsman clay bodies as a liner. Context: You can make your.., Industry can never make.., Catch Glaze Thursday 15th January 2026 Converting a glossy transparent glaze to a calcia matteA ten-minute video to give glaze nerds goose bumps!
Watch the G1214Z video to see me convert the G1214M cone 6 clear base into G1214Z cone 6 calcia matte using simple glaze chemistry and recipe logic. This first appeared in the Digitalfire desktop Insight instruction manual 30 years ago. It is an understatement to say that this process is interesting if you want to know more about glazes, their chemistry and recipe logic. Watch this video and see me adjust the recipe of my high-calcium transparent cone 6 glaze to convert it into a calcia matte. In an Insight-live.com account, the process is easy enough for anyone. We'll cut the Si:Al ratio, increase the CaO, maintain the thermal expansion for glaze fit and make the recipe shrinkage-adjustable using a mix of calcined kaolin and raw kaolin. We will even compare it with the High Calcium Semimatte from Mastering Glazes. Context: Two cone 6 matte.., Partially and fully opacified.., A hazard of using.., Calcia Matte, Converting G1214M Cone 6.. Wednesday 7th January 2026 This GA6-B glaze is better than beer bottle glass
Ceramic glazes, like this GA6-B, are actually just glass. But they are not like bottle glass. The latter is formulated to work well in forming machines (harden quickly), melt and stiffen quickly, have low melt viscosity and resist milkiness and crystallization on solidification. The chemistries to accomplish this have adequate resistance to leaching and adequate durability for a few uses. A stoneware glaze melt needs to be much more viscous (to stay put on vertical surfaces). And, it must have a lower thermal expansion (to match common clay bodies). And, it must resist crystallization much more (since it cools slowly). Fortunately, meeting these needs brings along big benefits: Greater durability, hardness and resistance to leaching. Stoneware glazes and bottle glass share a common trait: They have about the same amount of SiO2. But the similarity ends there, stoneware glazes have: Context: 3D-printing artifacts on a.., Meet two glazes at.., Regular bottles of beer.., v7 Classic beer bottle.., Food Safe, Beer Bottle Master Mold.. Wednesday 7th January 2026 Glaze dunking videos reveal the value of thixotropy
These videos from Eastfork Pottery demonstrate their use of thixotropic glaze slurries. Watch them to see how effective a highly gelled glaze is. It enables a quick dip, stays fluid while draining, gives even coverage and dries in seconds. These don't hard-pan or settle out in the bucket either. They work on porous or dense bisque. Almost any glaze can be thixotropic if you take the time to learn how to do it. The fast drying enables the use of twin running (or twin belt) foot wiper machines (best shown on these Instagram and Facebook videos). Context: Instagram Eastfork Pottery thixotropic.., Tiktok Eastfork Pottery thixotropic.., Facebook Eastfork Pottery thixotropic.., Eastfork Pottery, Thixotropy Thursday 11th December 2025 Glaze cracking during drying? Wash it off and then do this.
If your pottery glaze is doing on drying then it will crawl during firing. Wash it off, dry the ware. Then check the water content. If the glaze has worked fine in the past then it is likely going on too thick because the specific gravity is too high - just repeat cycles of adding a little water and dip testing (make it thixotropic if needed). But that was not the issue here. Glazes need clay to suspend and harden them, but too much clay means trouble. This was Ravenscrag Slip, a clay, being used pure as a cone 10R glaze. The glaze appeared to go in perfectly and it dried to the touch in ~20 seconds. But shrinkage continues after that, revealing after a couple of minutes. Fixing the issue was a matter of adding some roasted Ravencrag Slip to the bucket. That reduced the shrinkage and therefore the cracking. Any glaze containing excessive kaolin can be fixed the same way (trade some of the raw kaolin for calcined kaolin). Some glazes that contain plenty of clay also have bentonite - a simple fix for these is to simply remove the bentonite. Context: Calcined Kaolin, Calcination, Crawling Friday 5th December 2025 |
Plainsman Clays, 671 Industrial Ave, Medicine Hat, Alberta T1A 3L5
Phone: 403-527-8535
FAX: 403-527-7508